H: The Dilemma
Say you go to a pharmacy to place an order for a prescription, only to wonder why you're about to pay \$60 for twenty tablets when the generic version offers twice as many for $30. But then you wonder, is a generic pharmaceutical really the same as its private counterpart? Amazingly, the answer is yes...mostly..
He: A Legal Problem
The primary issue in creating a generic version of a private drug is making sure that the chemical composition of whatever compound is formed is not identical to that of any competitor'sThis mostly regards pharmaceuticals created in developed countries, where patent laws are well enforced.. On first glance, this seems very difficult to accomplish, since generic equivalents have to be made of the same active ingredient(s) as their private equivalents. Luckily, there is a powerful facet of organic chemistry that allows for the accomplishment of such a task:
Li: Chirality
A hugely important aspect in the understanding and appreciation of molecular structures, chirality refers to the asymmetric, non-superimposable relationship between two otherwise identical compounds. This is likely best understood with an example:
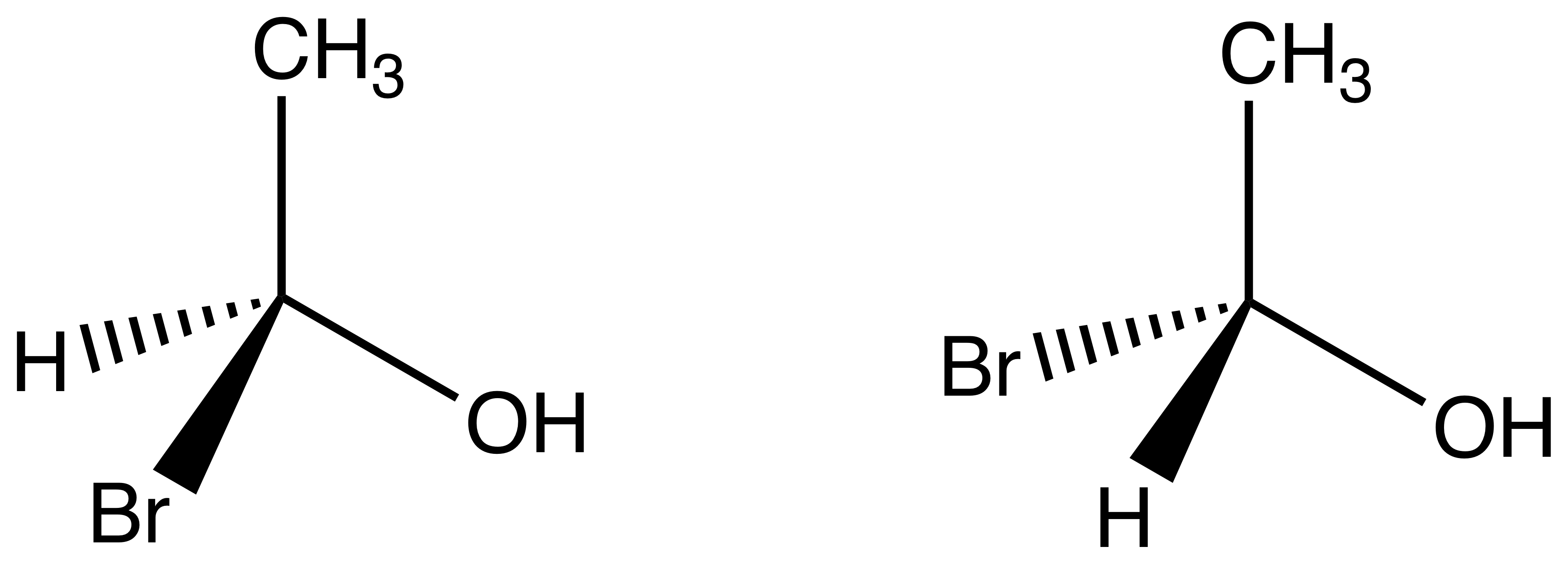
It is obvious that these two compounds are the same formulaically1-bromoethanol. But geometrically, they are very different! The two compounds' hydrogens and bromines are in different spatial configurations, arranged in such a way they never be superposed on one other, no matter how they are twisted or turned. Any two compounds with this property are known as stereoisomers. In this particular case, the two compounds are a special type of stereoisomers, known as enantiomersStereoisomers of a molecule are enantiomers if they have different configurations at each of their equivalent stereocenters, and if the molecule does not have an internal plane of symmetry. Enantiomers with an internal plane of symmetry are known as meso. Stereoisomers with different configurations at some, but not all, of their equivalent stereocenters are known as diastereomers..
Here's where it gets interesting.
See, enantiomers are identical with regard to their physical properties, except in one quality - optical rotation. Each enantiomer rotates polarized light by the same magnitude, but in opposite directions. This makes enantiomers fundamentally different compounds.
This means that one enantiomer can be produced without infringement on the registered sale of another, while providing the same physical propertiesOne enantiomer of a drug will be as medically useful as its counterpart, so long as there is no bias among drug receptors..
But as the margin notes, there is another problem here, because certain receptors in our bodies only respond to particular molecular geometries. In that case, what is a stereoisomer to do?
Be: The Hack
Recall the following fact:
Enantiomers rotate polarized light by the same magnitude, but in opposite directions.
This is the basis for how generic drugs can get away with it. The trick is to mix equal amounts of each enantiomer, creating a racemate - an optically inactiveOptically inactive mixture or compound does not rotate light - in this case, because the direction of rotation by each enantiomer cancels out. mixture of enantiomers. This way, a drug can be made that contains the active ingredient required for its operation, but is different enough in its physical properties and composition from a private counterpart to be competitively acceptable.
So, next time you're buying medication, save some money with the generic version and a doubled dosage!